Interruption of the inferior vena cava (IVC) for the prevention of PE was first performed in 1893 using surgical ligation [7]. Over the years, surgical interruption took many forms (ligation, plication, clipping, or stapling), but IVC thrombosis was a frequent complication after these procedures. Endovascular approaches to IVC interruption became a reality in 1967 after the introduction of the Mobin-Uddin filter [8].
I. INTRODUCTION
This practice parameter was revised collaboratively by the American College of Radiology (ACR) and the Society of Interventional Radiology (SIR).
Pulmonary embolism (PE) continues to be a major cause of morbidity and mortality in the United States. Estimates of the incidence of nonfatal PE range from 400,000 to 630,000 cases per year, and 50,000 to 200,000 fatalities per year are directly attributable to PE [1-4]. The current preferred treatment for deep venous thrombosis (DVT) and PE is anticoagulation. However, up to 20% of these patients will have recurrent PE despite adequate anticoagulation [3,5,6].
Interruption of the inferior vena cava (IVC) for the prevention of PE was first performed in 1893 using surgical ligation [7]. Over the years, surgical interruption took many forms (ligation, plication, clipping, or stapling), but IVC thrombosis was a frequent complication after these procedures. Endovascular approaches to IVC interruption became a reality in 1967 after the introduction of the Mobin-Uddin filter [8].
Many devices have since been developed for endoluminal caval interruption and currently several devices designed for permanent placements are commercially available in the United States. In addition to permanent IVC filters, retrievable IVC filters are also available. These filters can be left in place as a permanent implant but also can be removed when the indication for filter placement resolves. (For detailed information regarding each of these filters, the reader is referred to several reviews [9-23].) Selection of a device requires knowledge of the clinical settings in which filters are used, as well as an evaluation of the clot-trapping efficiency and structural integrity of the device, the occlusion rate of the IVC and access vein, the risk of filter movement and filter embolization, magnetic resonance imaging (MRI) compatibility of the device, and the ease of placement.
Placement of a caval filter can be performed as either an outpatient or inpatient procedure. Practically speaking, however, most filter placements will occur in the inpatient population because of ongoing medical therapy for acute thromboembolic disease or underlying illness.
The IVC should be assessed with imaging prior to placement of a filter, and the current preferred method is by vena cavography. Prior to filter selection and placement, the length and diameter of the infrarenal IVC should be assessed, the location and number of renal veins determined, IVC anomalies defined (e.g., duplication), and intrinsic IVC disease such as pre-existing thrombus or extrinsic compression excluded. If available, prior imaging studies (such as contrast enhanced computed tomography [CT] or MRI of the abdomen) may be used to evaluate the anatomy of the IVC (size, patency, and anatomical variants). The ideal location for filter placement for preventing lower extremity and pelvic venous thromboembolism is the infrarenal IVC. The apex or superior aspect of any filtration device should be at or immediately inferior to the level of the renal veins according to the manufacturer’s recommendations. In specific clinical circumstances other target locations may be appropriate.
Placement of a caval filter is commonly accomplished through right femoral or right internal jugular vein approaches; however, other peripheral (e.g., antecubital vein) and central venous access sites can be used. Filters can be placed in veins other than the IVC to prevent thromboembolism (an off-label indication). Implant sites have included iliac veins, subclavian veins, superior vena cava, and IVC (suprarenal and infrarenal). This paper only provides quality improvement guidelines for filter placement within the IVC due to the limited data available for implantation sites other than the IVC. The patient’s clinical condition, the type of filter available, the available access sites, and the expertise of the treating physician should always be considered when the decision to place an IVC filter has been made.
IVC filters labeled as retrievable by the U.S. Food and Drug Administration are also labeled for permanent placement. Retrievable filters may be placed with the intent of either temporary or permanent filtration. Removal of retrievable IVC filters may be accomplished in those cases where the indication was for prophylaxis and prevention of PE with temporary contraindication to anticoagulation. Filters placed with the intent of subsequent retrieval may be left in place permanently for any of several reasons (e.g., continuing need for filtration, thrombus on the filter, inability to retrieve the filter). Data for the feasibility of filter retrieval vary widely among devices and centers. Filters that are not retrieved function as permanent filters
II. DEFINITIONS [24,25]
For the purpose of this practice parameter, the following definitions apply:
Permanent placement – deployment in those situations where lifelong protection against thromboembolic episodes is needed.
Temporary placement – deployment in those situations where time-limited protection against thromboembolic episodes is needed.
Procedural success - deployment of a filter such that the filter is judged suitable for mechanical protection against PE.
Recurrent PE - pulmonary embolism occurring after filter placement and documented by pulmonary arteriography, cross-sectional imaging, or significant change in ventilation-perfusion (V/Q) lung scan indicative of recurrent PE, or autopsy.
IVC thrombotic occlusion - presence of an occluding thrombus in the IVC occurring after filter insertion and documented by ultrasound, CT, MRI, venography, or autopsy; this may be symptomatic or asymptomatic.
IVC penetration - penetration of the vein wall by filter hooks with transmural incorporation. For quality improvement reporting purposes, the definition of IVC penetration is filter strut or anchor devices extending more than 3 mm outside the wall of the IVC as demonstrated by CT, venography, or autopsy. Acute penetration occurring during placement of the filter is considered an insertion problem (see below).
Filter embolization – postdeployment movement of the filter or its components to a distant anatomic site completely out of the target zone.
Filter movement - a change in filter position compared to its deployed position (either cranial or caudal) of more than 2 cm as documented by plain film imaging, CT, or venography.
Filter fracture - any loss of a filter’s structural integrity (i.e., breakage or separation) documented by imaging or autopsy.
Insertion problems – malfunctions of the filter or deployment system such as incomplete filter opening, filter tilt more than 15 degrees from the IVC axis (e.g., non-self-centering filters), misplacement of filter outside the infrarenal IVC when the operator’s intent is to place the filter in the infrarenal IVC (e.g., when a portion of the filter is within one iliac vein), or prolapse of filter components. Filter malposition requiring surgical/ endovascular removal is considered an insertion problem complication.
Access site thrombus - occlusive or nonocclusive thrombus developing at the venotomy site after filter insertion, and documented by ultrasound or other imaging.
Access site complications with clinical sequelae - arteriovenous fistula, hematoma, or bleeding requiring transfusion, hospitalization (either admission or extended stay), or further treatment.
III. INDICATIONS [24-31]
-
Therapeutic (Documented Thromboembolic Disease)
-
Patients with evidence of pulmonary embolus or IVC, iliac, or femoral-popliteal DVT and one or more of the following:
-
Absolute or relative contraindication to anticoagulation.
-
Complication of anticoagulation.
-
Failure of anticoagulation.
-
Recurrent PE despite adequate therapy.
-
Inability to achieve/maintain adequate anticoagulation.
iii. Propagation/progression of DVT on therapeutic anticoagulation.
-
-
-
Massive pulmonary embolism with residual deep venous thrombus in a patient at risk for further PE.
-
Free-floating iliofemoral or inferior vena cava thrombus.
-
Severe cardiopulmonary disease and DVT (e.g., cor pulmonale with pulmonary hypertension).
-
-
Prophylactic (No Current Thromboembolic Disease)
-
Severe trauma without documented PE or DVT.
-
Closed head injury.
-
Spinal cord injury.
-
Multiple long bone or pelvic fractures.
-
-
High-risk patients (e.g., immobilized or in an intensive care unit).
-
-
Suprarenal Filter Placement
Suprarenal caval filter placement may be considered when any of the following situations exist in addition to the indications listed above.
-
Presence of IVC thrombus precluding placement of a filter in the infrarenal IVC.
-
Filter placement during pregnancy. Suprarenal placement is also appropriate in women of childbearing
age.
-
Thrombus extending above previously placed infarenal filter.
-
Gonadal vein thrombosis.
-
Anatomic variants: duplication of the IVC, low insertion of renal veins.
-
Significant extrinsic compression of the infrarenal IVC.
-
Intrinsic narrowing of the infrarenal IVC.
-
Patients with an intra-abdominal or pelvic mass who will undergo surgery and in whom operative IVC
mobilization is contemplated.
The IVC should be assessed with imaging prior to placement of a filter. The current preferred method is by vena cavography. Prior to filter selection and placement, the length and diameter of the suprarenal IVC should be assessed, the location and number of renal veins determined, the location and number of hepatic veins determined, the right atrium should be identified, IVC anomalies defined (e.g., duplication), and intrinsic IVC disease, such as pre-existing thrombus or extrinsic compression, excluded. If available, prior imaging studies (such as contrast enhanced CT or MRI of the abdomen) may be used to evaluate the anatomy of the IVC (size, patency, and anatomical variants). The anatomic considerations should be used in the final planning for filter placement and choice of device.
D. Filters Placed for Temporary Use and Possible Future Retrieval
Placement of filters for temporary use and possible future retrieval may be considered when any of the following situations exist in addition to the indications listed above.
1. PE and/or DVT and transient inability to anticoagulate.
-
Prophylactic prevention of PE in high-risk patients.
-
The use of retrievable filters should also be considered in pediatric and young adult patients, since the
long-term effects and durability of the devices are not precisely known. Currently there are no filters specifically designed for use in children. The safety and efficacy of vena cava filters in children have not been firmly established. Case reports and series have described the placement and removal of filters in children, but their long-term effect is unclear [32].
The threshold for these indications is 95%. When fewer than 95% of procedures are performed for these indications, the process of patient selection should be reviewed according to institutional policy.
IV. RELATIVE CONTRAINDICATIONS
A. Uncorrectable Severe Coagulopathy
B. Bacteremia or Untreated Infection
Clinical judgment should be applied in these situations, weighing the theoretical risk of implant infection versus the risk of pulmonary embolism.
V. QUALIFICATIONS AND RESPONSIBILITIES OF PERSONNEL
A. Physician
Examinations must be performed under the supervision of and interpreted by a physician who has the following qualifications:
-
Certification in Radiology or Diagnostic Radiology by the American Board of Radiology, the American Osteopathic Board of Radiology, the Royal College of Physicians and Surgeons of Canada, or the Collège des Médecins du Québec and must have demonstrated competency in vascular procedures under the supervision of an on-site qualified physician during the performance of at least 25 percutaneous vascular procedures of which at least five were as primary operator for IVC filter placement procedures.
or
-
Completion of a residency program approved by the Accreditation Council for Graduate Medical
Education (ACGME), the Royal College of Physicians and Surgeons of Canada (RCPSC), the Collège des Médecins du Québec, or the American Osteopathic Association (AOA) to include a minimum of 6 months training in the performance of percutaneous invasive vascular procedures and interventional radiology or vascular surgery that included the vascular aspects of interventional radiology, including at least 3 months in each of these subspecialty areas with at least 3 months of documented formal training in the performance of invasive catheter angiographic procedures, and must have demonstrated competency in vascular procedures under the supervision of an on-site qualified physician during the performance of at least 25 percutaneous vascular procedures of which at least five were as primary operator for IVC filter placement procedures.
or
-
In the absence of appropriate ACGME approved residency training as outlined in section V.A.2 above,
formal fellowship training in a Radiology Residency Review Committee (RRC) accredited vascular/interventional radiology fellowship program or other postgraduate training that included comparable instruction and experience in vascular interventional procedures. The physician must have at least 2 years experience with demonstrated competency as the primary operator in vascular interventional procedures under the supervision of an on-site qualified physician during which a minimum of 25 percutaneous vascular procedures, including a minimum of five IVC filter placement procedures, were performed with documented success and complication rates that meet the threshold criteria listed below (see section X).
4. Physicians meeting any of the qualifications in 1, 2, and 3 above must also have written substantiation that they are familiar with all of the following:
-
Indications and contraindications for the procedure.
-
Periprocedural and intraprocedural assessment, monitoring, and management of the patient and
potential complications.
-
Where applicable, pharmacology of moderate sedation medications and recognition and treatment of
adverse reactions and complications.
-
Appropriate use and operation of fluoroscopic and radiographic equipment, mechanical injectors,
digital image capture devices and electronic imaging systems.
-
Where applicable, principles of radiation protection, hazards of radiation, and radiation monitoring
requirements as they apply to both patients and personnel.
-
Where applicable, pharmacology of contrast agents and recognition and treatment of potential adverse
reactions.
-
Percutaneous needle and catheter introduction techniques.
-
Technical aspects of performing the procedure, including the use of alternative catheter and guidewire
systems, and filming sequences.
-
Anatomy, physiology, and pathophysiology of peripheral and central venous vasculature.
-
Postprocedure patient management, especially recognition and initial management of complications.
-
Postprocedure management of puncture sites.
-
Principles, operation, and imaging findings of vascular ultrasound, where applicable.
The written substantiation should come from the chief of interventional radiology, the chair of the department of radiology, or his or her designee at the institution in which the physician will be providing these services. Substantiation could also come from a prior institution in which the physician provided the services, but only at the discretion of the chair of the department of radiology or his or her designee who solicits the additional input.
Maintenance of Competence
Physicians must perform a sufficient number of filter placement procedures to maintain their skills, with acceptable success and complication rates as presented in this document (see section X). Continued competence should depend on participation in a quality improvement program that monitors these rates.
Continuing Medical Education
The physician’s continuing education should be in accordance with the ACR Practice Parameter for Continuing Medical Education (CME).
B. Qualified Medical Physicist
A Qualified Medical Physicist is an individual who is competent to practice independently in one or more of the subfields in medical physics. The American College of Radiology (ACR) considers certification, continuing education, and experience in the appropriate subfield(s) to demonstrate than an individual is competent to practice one or more of the subfields in medical physics and to be a Qualified Medical Physicist. The ACR strongly recommends that the individual be certified in the appropriate subfield(s) by the American Board of Radiology (ABR), the Canadian College of Physics in Medicine, or by the American Board of Medical Physics (ABMP).
A Qualified Medical Physicist should meet the ACR Practice Parameter for Continuing Medical Education (CME). (ACR Resolution 17, 1996 – revised in 2012, Resolution 42)
The appropriate subfield of medical physics for this practice parameter is Diagnostic Medical Physics. (Previous medical physics certification categories including Radiological Physics, Diagnostic Radiological Physics, and Diagnostic Imaging Physics are also acceptable.)
C. Registered Radiologist Assistant
A registered radiologist assistant is an advanced level radiographer who is certified and registered as a radiologist assistant by the American Registry of Radiologic Technologists (ARRT) after having successfully completed an advanced academic program encompassing an ACR/ASRT (American Society of Radiologic Technologists) radiologist assistant curriculum and a radiologist-directed clinical preceptorship. Under radiologist supervision, the radiologist assistant may perform patient assessment, patient management and selected examinations as delineated in the Joint Policy Statement of the ACR and the ASRT titled “Radiologist Assistant: Roles and Responsibilities” and as allowed by state law. The radiologist assistant transmits to the supervising radiologists those observations that have a bearing on diagnosis. Performance of diagnostic interpretations remains outside the scope of practice of the radiologist assistant. (ACR Resolution 34, adopted in 2006)
D. RadiologicTechnologist
-
The technologist, together with the physician and nursing personnel, should be responsible for patient comfort and safety. The technologist should be able to prepare and position2 the patient for the filter placement procedure and, together with the nurse, monitor the patient during the procedure. The technologist should obtain the imaging data in a manner prescribed by the supervising physician. If intravenous contrast material is to be administered, qualifications for technologists performing intravenous injection should be in compliance with current ACR policy3 and existing operating procedures or manuals at the facility. The technologist should also perform the regular quality control testing of the equipment under the supervision of the physicist.
-
Technologists should be certified by the American Registry of Radiologic Technologists (ARRT) or have an unrestricted state license with documented training and experience in the imaging modality used for the imaging-guided procedure.
E. Nursing Services
Nursing services, when deemed appropriate by the performing physician, are an integral part of the team for preprocedure and postprocedure patient management and education and are recommended in monitoring the patient during the procedure.
VI. SPECIFICATIONS OF THE EXAMINATION
There are several technical requirements to ensure safe and successful filter placement procedures. These include adequate angiographic equipment and institutional facilities, physiologic monitoring equipment, and support personnel.
A. EquipmentandFacilitiesforFilterPlacement
The following are considered the minimum equipment requirements for performing vena cavagrams and filter placement. In planning facilities for IVC placement, equipment and facilities more advanced than those outlined below may be desired to produce higher quality studies with reduced risk and time of study. The facility should include at a minimum:
2The American College of Radiology approves of the practice of certified and/or licensed radiologic technologists performing fluoroscopy in a facility or department as a positioning or localizing procedure only, and then only if monitored by a supervising physician who is personally and immediately available*. There must be a written policy or process for the positioning or localizing procedure that is approved by the medical director of the facility or department/service and that includes written authority or policies and processes for designating radiologic technologists who may perform such procedures. (ACR Resolution 26, 1987 – revised in 2007, Resolution 12-m)
*For the purposes of this parameter, “personally and immediately available” is defined in manner of the “personal supervision” provision of CMS—a physician must be in attendance in the room during the performance of the procedure. Program Memorandum Carriers, DHHS, HCFA, Transmittal B-01-28, April 19, 2001.
3See the ACR–SPR Practice Parameter for the Use of Intravascular Contrast Media.
-
A high-resolution image receptor, preferably with a 28 to 40 cm field of view, and an imaging chain with either standard angiographic filming capabilities including serial 14-inch film changers, or (preferably) a digital imaging system with a minimum 1,024-image matrix. Digital angiographic systems are preferred, as they allow for reduced volumes of contrast material and reduced examination times. Images are acquired and stored either on conventional film or digitally on computerized storage media. Imaging and image recording must be consistent with the as-low-as-reasonably-achievable (ALARA) radiation safety guidelines. The use of cineradiography or small field mobile image intensifiers is inappropriate for the routine recording of the venacavagram and IVC placement, because these methods cause an unacceptably high patient and operator radiation dose. Use of last image-hold and pulsed fluoroscopy are recommended for dose reduction.
-
Adequate angiographic supplies such as catheters, guidewires, needles, and introducer sheaths.
-
An angiographic injector capable of varying injection volumes and rates with appropriate safety
mechanisms to prevent overinjection.
-
An angiography suite that is large enough to allow easy transfer of the patient from the bed to the table
and allow room for the procedure table, monitoring equipment, and other hardware such as intravenous pumps, respirators, anesthesia equipment, and oxygen tanks. Ideally, there should be adequate space for the operating team to work unencumbered on either side of the patient and for the circulation of other technical staff in the room without contaminating the sterile conditions.
-
An area within the institution appropriate for patient preparation prior to the procedure and for observation of patients after the procedure. This might be within the radiology department, a short-stay unit, a routine nursing unit, or a postanesthesia care unit. At this location, there should be personnel to provide care as outlined below (Patient Care), and there should be immediate access to emergency resuscitation equipment.
-
Physiologic Monitoring and Resuscitation Equipment
-
Equipment should be present in the procedure suite to allow for monitoring the patient’s heart rate, cardiac rhythm, and blood pressure. For facilities using moderate sedation, a pulse oximeter monitor should be available (see the ACR–SIR Practice Parameter for Sedation/Analgesia).
-
Appropriate emergency equipment and medications must be immediately available to treat adverse reactions associated with administered medications and/or procedural complications. The equipment should be maintained and medications inventoried for drug expiration dates on a regular basis. The equipment, medications, and other emergency support must also be appropriate for the range of ages and sizes in the patient population.
-
-
Support Personnel
-
Radiologic technologists properly trained in the use of the angiographic equipment should assist in performing and imaging the procedure. They should demonstrate appropriate knowledge of patient positioning, angiographic image recording, angiographic contrast injectors, angiographic supplies including IVC filters, and the physiologic monitoring equipment. Certification as a vascular and interventional radiologic technologist is one measure of appropriate training. The technologist should be trained in basic cardiopulmonary resuscitation and in the function of the resuscitation equipment.
-
If the patient does not receive sedation for the procedure, one of the staff assisting the procedure should be assigned to periodically assess the patient’s status. In cases where moderate sedation is used in adults, light or moderate sedation is used in children, or the patient is critically ill, an experienced licensed provider should be present whose primary responsibility is monitoring the patient’s vital signs, sedation state, and level of comfort/pain. This person should maintain a record of the patient's vital signs, the time and dose of medications given, and other pertinent information (see the ACR–SIR Practice Parameter for Sedation/Analgesia).
-
D. Acute Care Support
Although surgical or other emergency treatment is needed infrequently for serious complications after filter placement procedures, there should be prompt access to surgical and interventional equipment and to specialists familiar with the management of patents with complications in the unlikely event of a life-threatening complication.
E. Patient Care
For additional information see the ACR–SIR–SNIS–SPR Practice Parameter for Interventional Clinical Practice and Management.
1. Preprocedure care
For elective filter placement, the following should be documented:
-
Clinically significant history, including indications for the procedure.
-
Clinically significant physical or diagnostic examination findings, including clinical or medical
conditions that may necessitate specific care, such as preprocedure antibiotics and other measures.
-
Clinically indicated laboratory evaluation including, but not limited to, coagulation factors, creatinine,
white blood cell count, and previously obtained cultures.
-
Preprocedure documentation should conform to the requirements of the ACR–SIR–SPR Practice
Parameter for the Reporting and Archiving of Interventional Radiology Procedures.
Informed consent must be in compliance with all state laws and the ACR–SIR Practice Parameter on Informed Consent for Image-Guided Procedures.
For emergency procedures, a note should be written summarizing the indication for the study, the pertinent history and physical findings, if available, and the proposed procedure.
-
Procedural care
-
Adherence to the Joint Commission’s Universal Protocol for Preventing Wrong Site, Wrong
Procedure, Wrong Person SurgeryTM is required for procedures in non-operating room settings, including bedside procedures.
The organization should have processes and systems in place for reconciling differences in staff responses during the “time out.” -
All patients should have cardiac monitoring continuously during the procedure with intermittent blood pressure monitoring. A record of vital signs should be maintained.
-
All patients should have intravenous access for the administration of fluids and medications as needed.
-
If the patient is to receive sedation for the procedure, pulse oximetry should be used. A registered nurse or other appropriately trained personnel should be present, and his/her primary responsibility should be to monitor the patient. A record should be kept of medication doses and times of administration. See the ACR–SIR Practice Parameter for Sedation/Analgesia.
-
-
Postprocedure care
-
All patients should be at bed rest and observed in the initial postprocedure period. The length of this
period of bed rest will depend on the site and size of the venotomy and the patient’s medical
condition.
-
During the initial postprocedure period, skilled nurses or other appropriately trained personnel should
periodically monitor the puncture site.
-
Initial ambulation of the patient must be carefully supervised. The puncture site stability and
independent patient function and mobility must be assured.
-
The operating physician or a qualified designee should evaluate the patient after the procedure, and
these findings should be summarized in a progress note. If moderate sedation was administered prior
-
to and during the procedure, complete recovery from sedation must be documented. The physician or designee should be available for continuing care during hospitalization and after discharge. The designee may be another physician or a nurse. See the ACR–SIR Practice Parameter for Sedation/Analgesia.
F. Selection Criteria for Short-Term Observation
The duration of postprocedure observation must be individualized. IVC filter placement can be performed on some patients with a short period of postprocedure observation (less than 6 hours) prior to discharge to home; others require overnight care. Short-term observation should only be considered when all the following conditions can be met:
-
Those patients capable of independent ambulation prior to the procedure demonstrate stable independent ambulation after the procedure. Nonambulatory patients have adequate assistance after discharge to provide care as needed.
-
The patient is capable of following instructions and detecting changes in symptomatology. Alternatively, patients with impaired mental or neurologic status should have adequate assistance after discharge to provide care as needed.
-
The patient is provided with instructions on how to recognize potential complications and how to obtain medical assistance in the event of such complication. A responsible adult is also provided with information regarding recognition of potential complications and is available to transport the patient and be in attendance during the initial night after discharge.
-
The patient is free of concurrent serious medical illness that might contribute to a significantly increased risk of complication.
-
The patient has recovered from the effects of the sedation.
G. Relative Contraindications to Short-Term Observation
Several factors must be considered when determining the length of postprocedure skilled nursing care. Some of the relative contraindications to short-term observation are listed below.
-
Patients with significant risk of contrast media-associated nephrotoxicity that might be prevented by hospitalization and intravenous hydration.
-
Patients with coagulopathies or electrolyte abnormalities that require correction should be hospitalized until stable.
-
Insulin-dependent diabetics who have labile serum glucose levels in the periprocedural period should be hospitalized until stable.
-
Complications occurring during or after IVC filter placement, including large hematoma, anuria, and persistent nausea and vomiting should prompt observation until symptoms resolve.
-
Patients who exhibit hemodynamic instability or significant dysrhythmia during or after the procedure should be hospitalized until stable.
-
Patients who live alone.
-
Patients with concurrent serious medical illness that might contribute to a significantly increased risk of
complication should be hospitalized until stable.
-
Patients with impaired mental or neurologic status who do not have adequate assistance to provide care as
needed should be hospitalized until appropriate assistance is available or no longer required.
The decision for short-term or longer-term postprocedure observation must be individualized, and a patient's care may vary from the above criteria for sound clinical reasons. The decision in each case must be made by the physician who performed the procedure and the referring physician after review of all pertinent data.
VII. DOCUMENTATION
Reporting should be in accordance with the ACR–SIR Practice Parameter for the Reporting and Archiving of Interventional Radiology Procedures.
VIII. RADIATION SAFETY IN IMAGING
Radiologists, medical physicists, registered radiologist assistants, radiologic technologists, and all supervising physicians have a responsibility for safety in the workplace by keeping radiation exposure to staff, and to society as a whole, “as low as reasonably achievable” (ALARA) and to assure that radiation doses to individual patients are appropriate, taking into account the possible risk from radiation exposure and the diagnostic image quality necessary to achieve the clinical objective. All personnel that work with ionizing radiation must understand the key principles of occupational and public radiation protection (justification, optimization of protection and application of dose limits) and the principles of proper management of radiation dose to patients (justification, optimization and the use of dose reference levels) http://www- pub.iaea.org/MTCD/Publications/PDF/Pub1578_web-57265295.pdf.
Nationally developed guidelines, such as the ACR’s Appropriateness Criteria®, should be used to help choose the most appropriate imaging procedures to prevent unwarranted radiation exposure.
Facilities should have and adhere to policies and procedures that require varying ionizing radiation examination protocols (plain radiography, fluoroscopy, interventional radiology, CT) to take into account patient body habitus (such as patient dimensions, weight, or body mass index) to optimize the relationship between minimal radiation dose and adequate image quality. Automated dose reduction technologies available on imaging equipment should be used whenever appropriate. If such technology is not available, appropriate manual techniques should be used.
Additional information regarding patient radiation safety in imaging is available at the Image Gently® for children (www.imagegently.org) and Image Wisely® for adults (www.imagewisely.org) websites. These advocacy and awareness campaigns provide free educational materials for all stakeholders involved in imaging (patients, technologists, referring providers, medical physicists, and radiologists).
Radiation exposures or other dose indices should be measured and patient radiation dose estimated for representative examinations and types of patients by a Qualified Medical Physicist in accordance with the applicable ACR Technical Standards. Regular auditing of patient dose indices should be performed by comparing the facility’s dose information with national benchmarks, such as the ACR Dose Index Registry, the NCRP Report No. 172, Reference Levels and Achievable Doses in Medical and Dental Imaging: Recommendations for the United States or the Conference of Radiation Control Program Director’s National Evaluation of X-ray Trends. (ACR Resolution 17 adopted in 2006 – revised in 2009, 2013, Resolution 52).
IX. QUALITY CONTROL AND IMPROVEMENT, SAFETY, INFECTION CONTROL, AND PATIENT EDUCATION
Policies and procedures related to quality, patient education, infection control and safety should be developed and implemented in accordance with the ACR Policy on Quality Control and Improvement, Safety, Infection Control, and Patient Education appearing under the heading Position Statement on QC & Improvement, Safety, Infection Control, and Patient Education on the ACR web site (http://www.acr.org/guidelines).
These data should be utilized in conjunction with the thresholds described in section X below to assess filter placement procedural efficacy and complication rates, and to trigger institutional review when these thresholds are exceeded.
X. QUALITY IMPROVEMENT
A. Success Rates and Thresholds
While practicing physicians should strive to achieve perfect outcomes (e.g., 100% success, 0% complications), in practice, all physicians will fall short of this ideal to a variable extent. Thus indicator thresholds may be used to assess the efficacy of ongoing improvement programs.
For the purpose of these practice parameters, a threshold is a specific level of an indicator that should prompt a review. Individual complications may also be associated with complication-specific thresholds. When measures such as indications or success rates fall below a minimum threshold, or when complication rates exceed a maximum threshold, a review should be performed to determine causes and to implement changes, if necessary. Thresholds may vary from those listed here; for example, patient referral patterns and selection factors may dictate a different threshold value for a particular indicator at a particular institution. Thus, setting universal thresholds is very difficult, and each department is urged to alter the thresholds as needed to higher or lower values to meet its own quality improvement program needs.
Complications can be stratified on the basis of outcome. Major complications result in admission to a hospital for therapy (for outpatient procedures), an unplanned increase in the level of care, prolonged hospitalization, permanent adverse sequelae, or death. Minor complications result in no sequelae; they may require nominal therapy or a short hospital stay for observation (generally overnight). (See Appendix A.) The complication rates and thresholds below refer to major complications.
It is expected that the technical success for percutaneously placed IVC filters will be 97% or better in experienced hands. Therefore, the proposed threshold for review of technical failures should be 3%.
B. Complication Rates and Thresholds
1. Complications
Each currently available filter has been extensively studied as part of the FDA approval process. Few comparative studies have been completed evaluating all filters in one project, and those that have done so have been retrospective analyses. Complication rates are highly variable depending on the filter being studied. For simplicity, these practice parameters do not suggest threshold rates for each individual filter; rather, filtration devices are considered as a group.
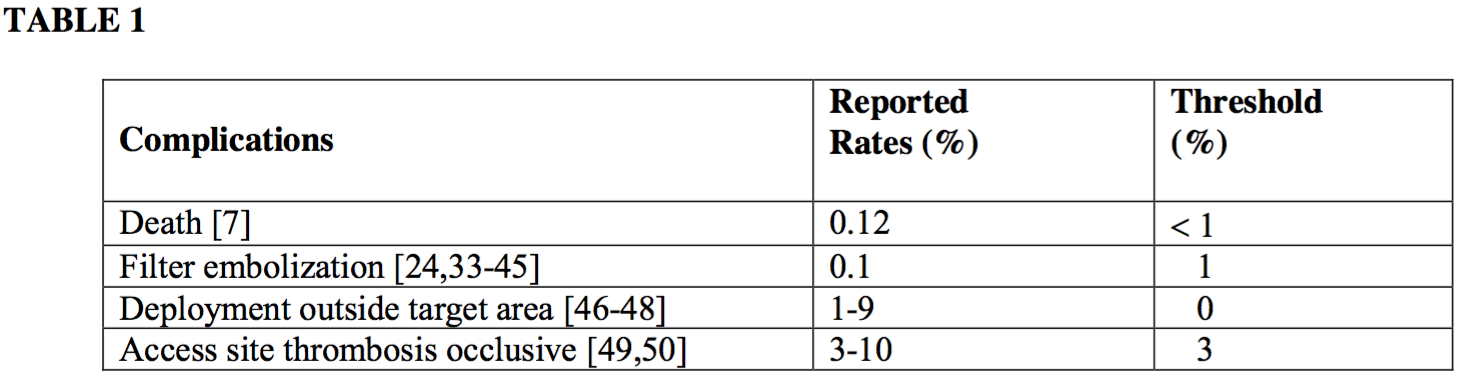
Published rates for individual types of complications are highly dependent on patient selection and are in some cases based on series comprising several hundred patients, which is a volume larger than most individual practitioners are likely to treat. It is also recognized that a single complication can cause a rate to cross above a complication-specific threshold when the complication occurs within a small patient volume (e.g., early in a quality improvement program).
2. Other trackable events
Because an IVC filter may be implanted as a permanent device (if not retrieved) and can be used in relatively young patients, several other trackable parameters when observed are appropriate to record in a quality improvement program. The following events may or may not be clinically significant in a particular patient. For this reason, thresholds for these events are not included in this document.
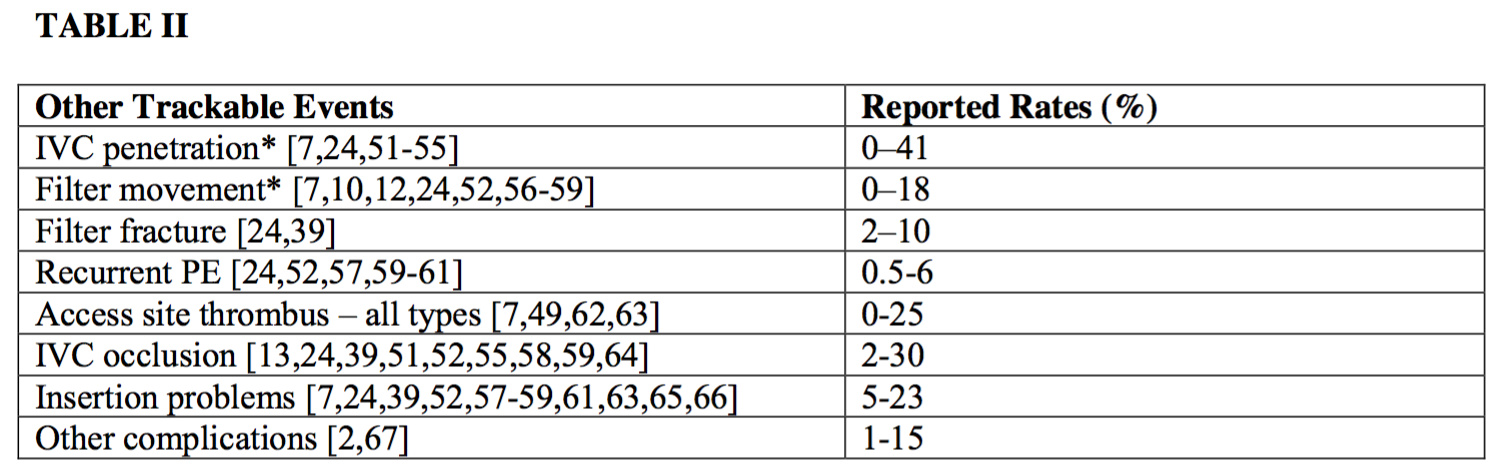
*Clinically significant penetration and movement are felt to be rare. The rate of clinically significant penetration has been reported to be 0.4% [68] but is not precisely defined in the literature.
ACKNOWLEDGEMENTS
This guideline was revised according to the process described under the heading The Process for Developing ACR Practice Guidelines and Technical Standards on the ACR web site (http://www.acr.org/guidelines) by the Guidelines and Standards Committee of the Commission on Interventional and Cardiovascular Radiology, in collaboration with the SIR.
Some references which i kept at all in this. You should read original article.
Duc Tin surgical clinic.
Tin tức liên quan

Performance diagnostique de l’interféron gamma dans l’identification de l’origine tuberculeuse des pleurésies exsudatives

A Mixed Phenotype of Airway Wall Thickening and Emphysema Is Associated with Dyspnea and Hospitalization for Chronic Obstructive Pulmonary Disease.

Radiological Approach to Asthma and COPD-The Role of Computed Tomography.

Significant annual cost savings found with UrgoStart in UK and Germany

Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Phone: (028) 3981 2678
Mobile: 0903 839 878 - 0909 384 389







